Which Group Tends To Form 1 Ions - Study with quizlet and memorize flashcards containing terms like which group tends to. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which group tends to not form ions or react? Which periodic group forms +1 ;
Which group tends to not form ions or react? Which periodic group forms +1 ; The group that tends to form 1+ ions is group 1, which consists of alkali metals such. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Study with quizlet and memorize flashcards containing terms like which group tends to.
Which periodic group forms +1 ; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which group tends to not form ions or react? The charge on a ion indicates the no. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Study with quizlet and memorize flashcards containing terms like which group tends to. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions.
Naming Simple Ionic Compounds Pathways to Chemistry
The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The charge on a ion indicates the no. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Which group tends.
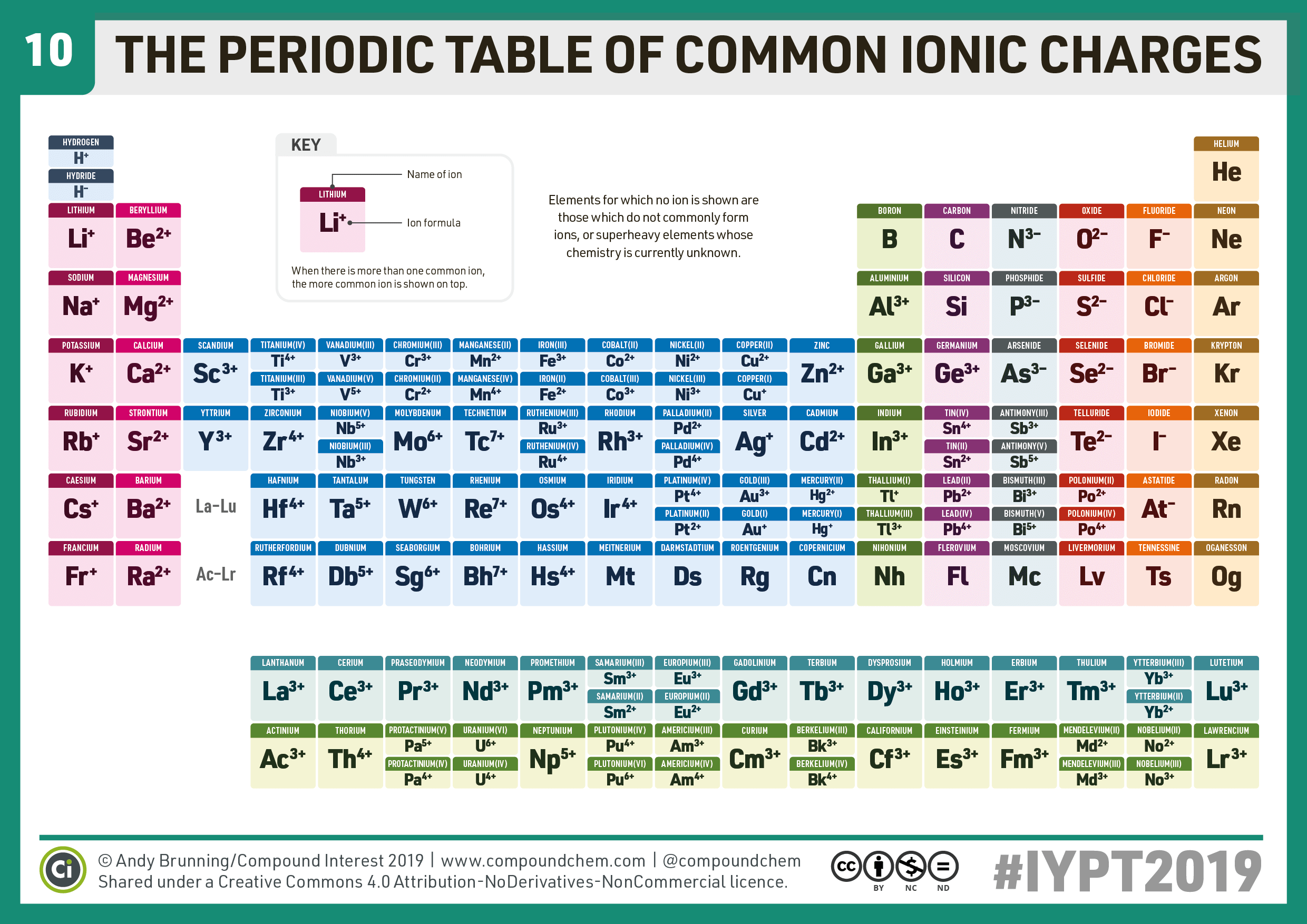
Compound Interest 10 Periodic Table of Common Ions
Study with quizlet and memorize flashcards containing terms like which group tends to. Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which periodic group forms +1 ;
Do Metals Form Positive Or Negative Ions
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which group tends to not form ions or react? Which periodic group forms +1 ; Study with quizlet and memorize flashcards containing terms like which group tends to.
Charge Of Ions Calculator
Which periodic group forms +1 ; Study with quizlet and memorize flashcards containing terms like which group tends to. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The elements in group 1 of the periodic table (alkali metals) tend to form +1.
metals tend to form what kind of ions Lombardi Bothe1936
The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Study with quizlet and memorize flashcards containing terms like which group tends to. Which periodic group forms +1 ; Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. Which group tends to not form.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Study with quizlet and memorize flashcards containing terms like which group tends to. The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. The charge on a ion indicates the no. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Group 1 metals, the alkali metals, have.
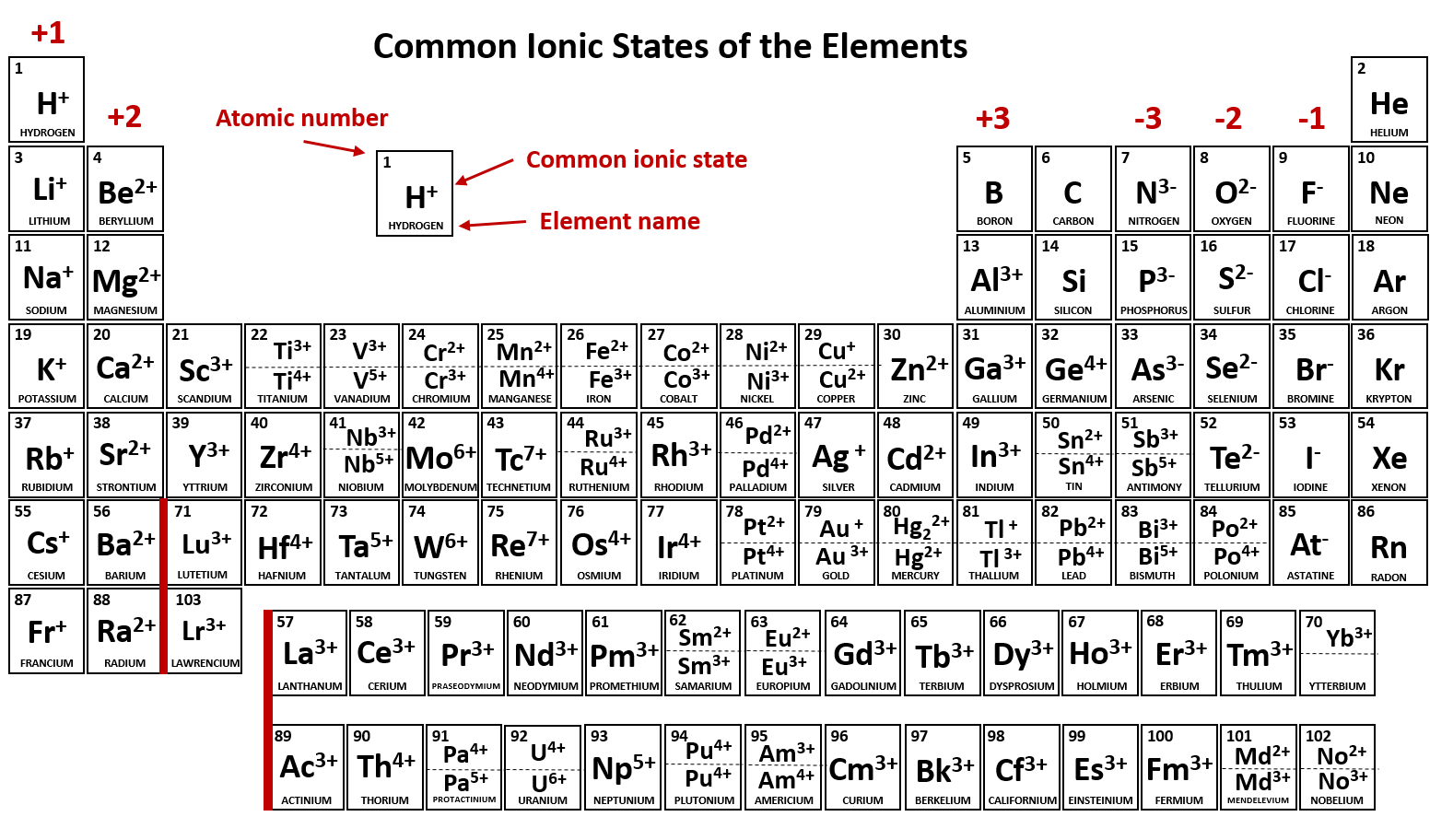
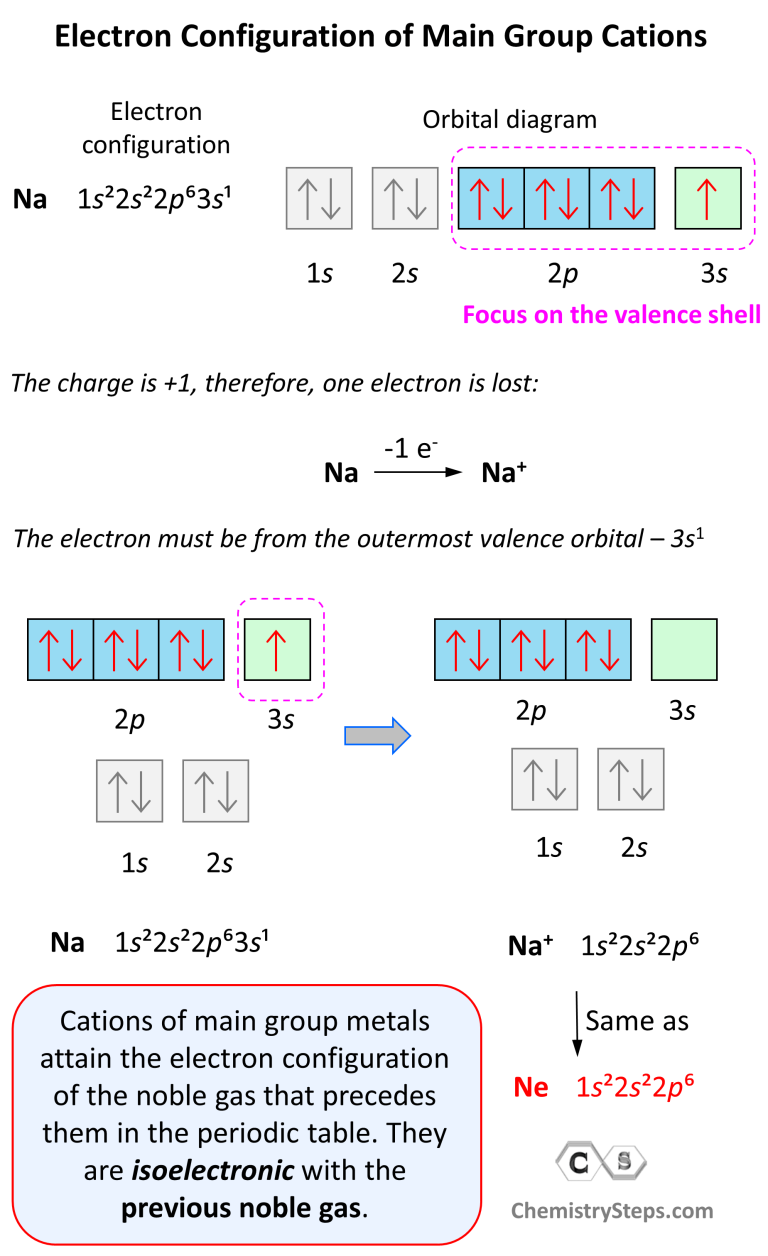
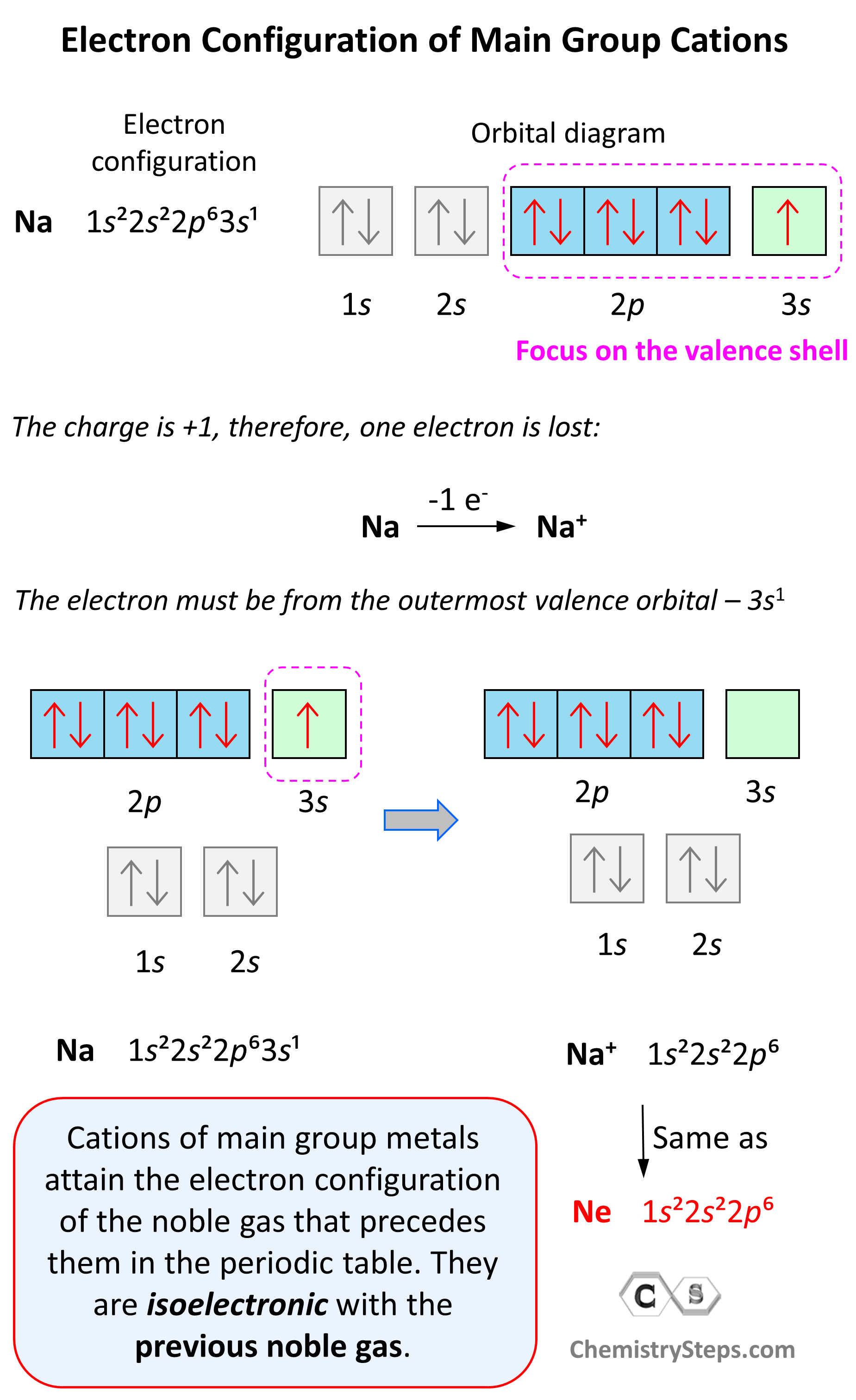
Electron Configurations of Ions Chemistry Steps
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Which group tends to not form ions or react? Which periodic group forms +1 ; The charge on a ion indicates the no. The group that tends to form 1+ ions is group 1, which consists of alkali metals such.
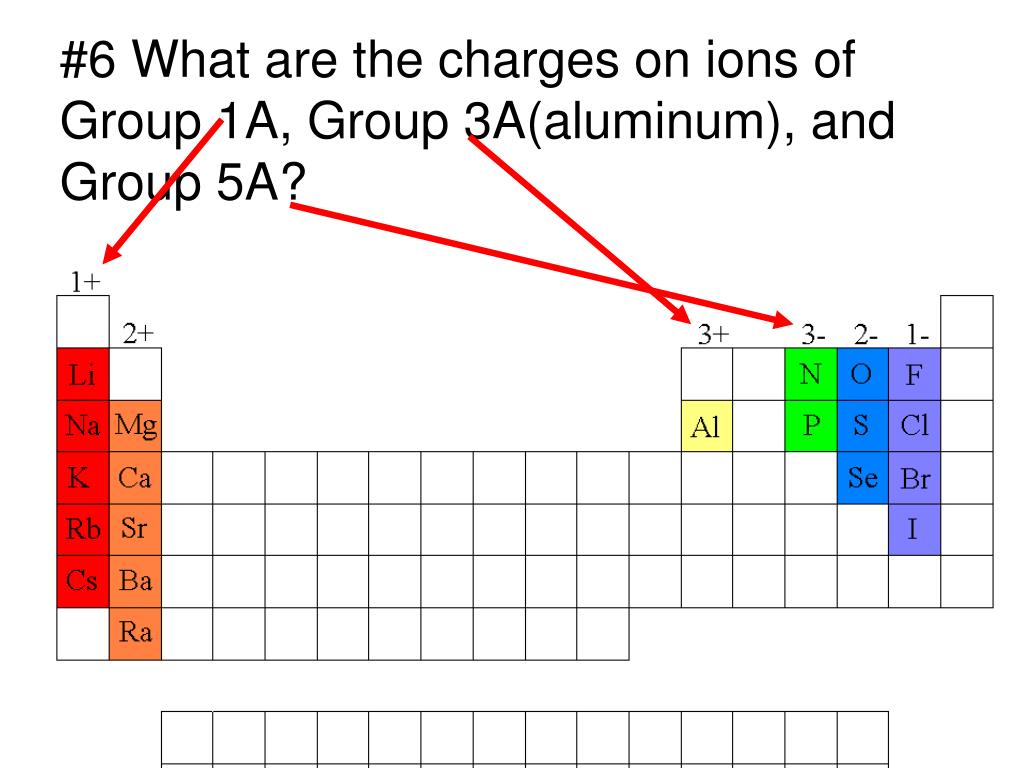
PPT 1 Name the ions formed by these elements and classify them as
Which group tends to not form ions or react? Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. The charge on a ion indicates the no. Study with quizlet and memorize flashcards containing terms like which group tends to. Group 1 metals, the alkali metals, have the 1 valence electron and thus form.
Electron Configurations of Ions Chemistry Steps
Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +. Study with quizlet and memorize flashcards containing terms like which group tends to. Which periodic group forms +1 ; The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. The charge on a ion indicates the no.
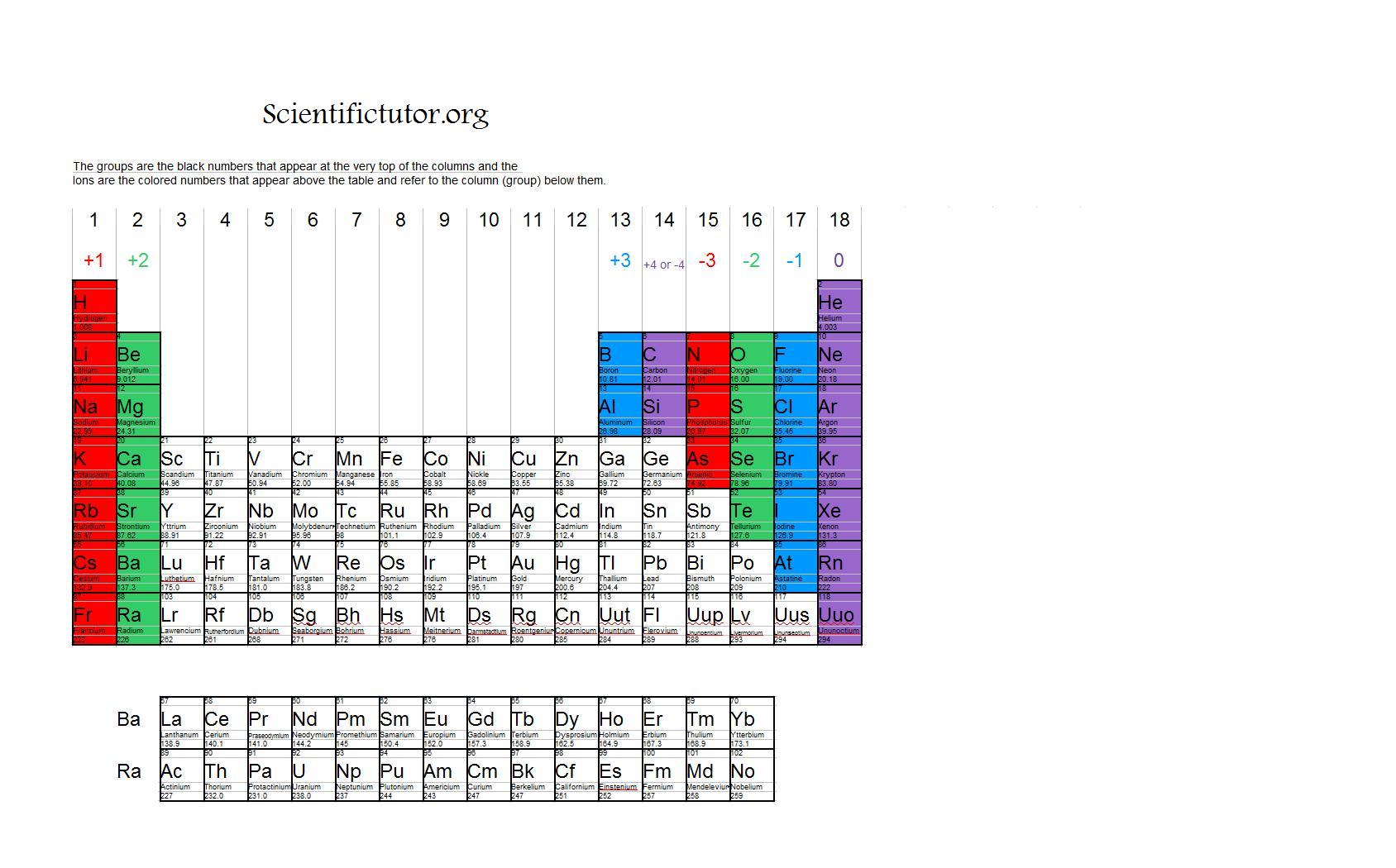
Chem Ions Scientific Tutor
Which periodic group forms +1 ; The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The group that tends to form 1+ ions is group 1, which consists of alkali metals such. Which group tends.
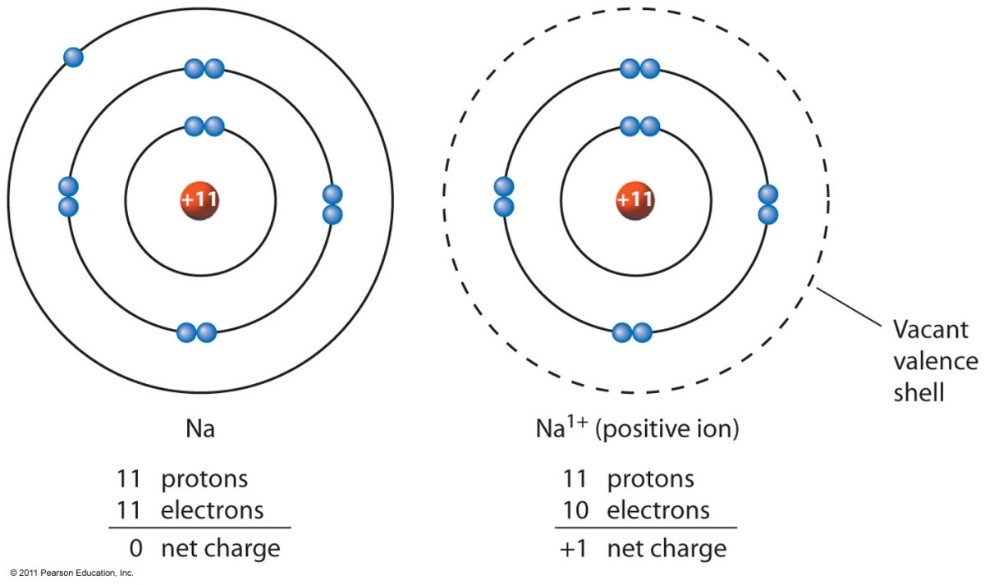
Group 1 Metals, The Alkali Metals, Have The 1 Valence Electron, And Thus Form M +.
The elements in group 1 of the periodic table (alkali metals) tend to form +1 ions. Which periodic group forms +1 ; Study with quizlet and memorize flashcards containing terms like which group tends to. Which group tends to not form ions or react?
The Charge On A Ion Indicates The No.
Group 1 metals, the alkali metals, have the 1 valence electron and thus form $$ m ^ {+}$$. The group that tends to form 1+ ions is group 1, which consists of alkali metals such.